
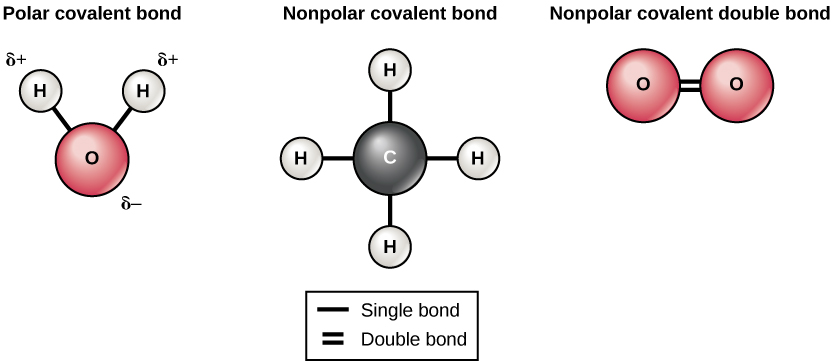
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. The ribose is located near Ala753, which together with Ala752 may provide the space necessary for the ligand to bind. The white spheres of these clusters are closest to the purple spheres. One of the terminal oxygen atoms of the phosphate group of c-di-AMP forms a hydrogen bond with the hydroxyl group of Tyr749, while the other terminal oxygen interacts with the Ser756 side chain through a water molecule.
#Non cyclic amp molecule after dissolved in water plus#
One of the K superscript plus purple spheres is surrounded by four of the red and white clusters. Cyclic adenosine monophosphate (cAMP, cyclic AMP or 3-5-cyclic adenosine monophosphate) is a molecule that is important in many biological processes it is derived from adenosine triphosphate. Two of the green C l superscript minus spheres are surrounded by three of the red and white clusters, with the red spheres closer to the green spheres than the white spheres. The effect on development appears to depend only on hydrolysis of extracellular cyclic AMP and not on other properties of the phosphodi-esterase molecule. A red sphere in one of these clusters is labeled O. In the picture attached, the bond that's lost is shown by the blue line between the Phosphorus (P) atom and the oxygen (O) atom of the main aromatic ring. The cyclic AMP serves as a messenger in a variety of biological processes.

Outside of this cluster of spheres are seventeen clusters of three spheres, which include one red and two white spheres. According to the recent study by Whiteley et al. A cyclic AMP, when dissolved in water loses the bond between its Phosphorus (P) atom and its main aromatic ring. The diagram shows eight purple spheres labeled K superscript plus and eight green spheres labeled C l superscript minus mixed and touching near the center of the diagram. Water molecules in front of and behind the ions are not shown. Draw the noncyclic amp molecule after it has dissolved in. What does cyclic AMP do in the body The hydroxyl ion donates an electron to chlorophyll and the OH resulting from this forms water and oxygen. Low-molecular weight amines are generally water-soluble. The cyclic AMP is a messenger involved in many biological processes. When light energy reaches the reaction centre a pair of. In the pictures attached, is shown in red the bond that's lost. The polar water molecules are attracted by the charges on the K + and Cl − ions. When a cyclic AMP is dissolved in water it loses its bond between its P atom and its main aromatic ring. In each case, the wire leads from the wall to the beaker and is split resulting in two ends.\): As potassium chloride (KCl) dissolves in water, the ions are hydrated. Each has a wire plugged into a wall outlet. This diagram shows three separate beakers. childtime corporate office bob livingston drummer santana kelly robinson mathew baynton wife Open menu.

The conductivity of an electrolyte solution is related to the strength of the electrolyte. Solutions of electrolytes contain ions that permit the passage of electricity. Quantification of intracellular cAMP levels remains an important methodology in molecular pharmacological studies of GPCRs. Biology Article Cyclic And Non Cyclic Photophosphorylation Cyclic And Non Cyclic Photophosphorylation We all are well aware of the complete process of photosynthesis. What is the mass of sodium chloride used to make a 100 mL solution at a concentration of 1.5 M Your solution mus. Detail the conversion into units of pounds per liter, and provide the density. So, when the functional group in non cyclic amp (Adenosine monophosphate, it is a nucleotide and also known as 5'-adenylic acid,) molecule contains acidic hydrogens, it means it has the ability to apply the acidic property on the molecule. Q: The density of table salt is 2.16 g/cm 3. Acidic hydrogens shows that the hydrogen atom present in them have ability to release positive ion that is H. \): Solutions of nonelectrolytes, such as ethanol, do not contain dissolved ions and cannot conduct electricity. Sutherland in 1958 for which he received a Nobel prize. Cyclic adenosine monophosphate (cAMP) is a common second messenger that is regulated by the activation of G protein-coupled receptors (GPCRs) and mediates numerous biological responses. Q: Draw the noncyclic AMP molecule after it has dissolved in water.


 0 kommentar(er)
0 kommentar(er)
